April 30, 2024, Bortezomib for injection receives the approval letter by NMPA, formally approved for marketing in China, with independent research and development by Kindos Pharmaceuticals Co., Ltd (Hereinafter referred to ”Kindos”) .
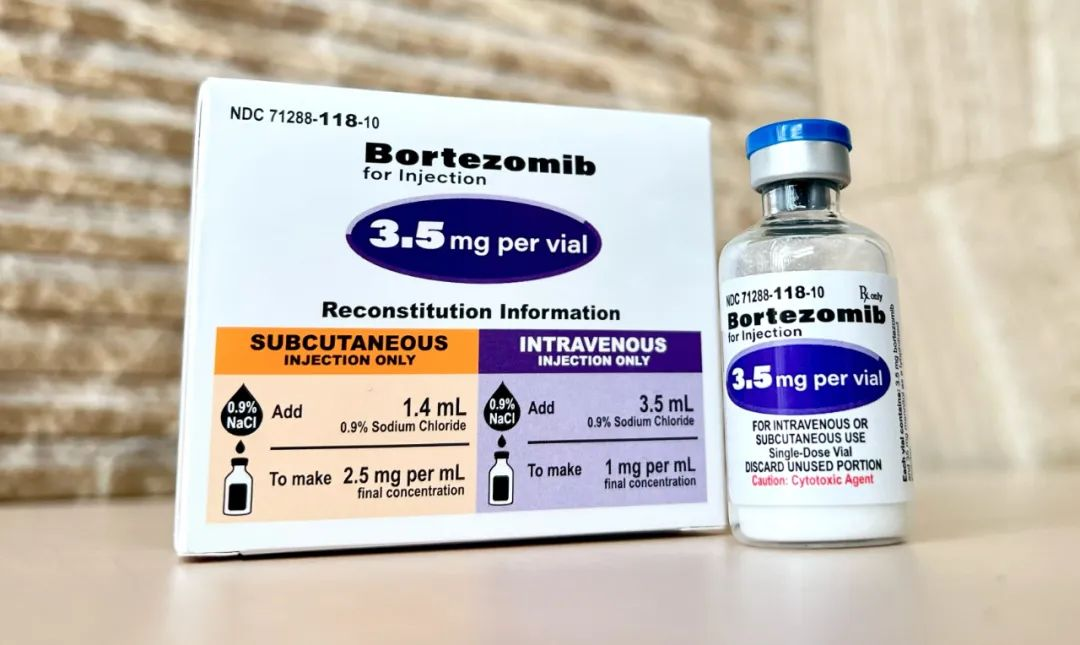
Bortezomib is the first newly synthetic proteasome competitive inhibitor for clinical use worldwide. It is the first-line potent targeted drug for the treatment of multiple myeloma and is also mostly used in the clinical treatment of mantle cell lymphoma. This product is one of the key products submitted to both NMPA and US FDA. Data shows the U.S. market size of Bortezomib for Injection in 2023 is nearly USD 100 million, and Chinese market size is about RMB 400 to 500 million.
Bortezomib for injection, independently researched and developed by Kindos, was approved by US FDA in 2022 and has been sold in US since the next day of approval date. And the export sales shows steady growth.The approval by NMPA continually widen the pipelines of drug products submitted and marketed in both China and US, meanwhile, it provides more choices of high quality drug products for China clinical requests.






